
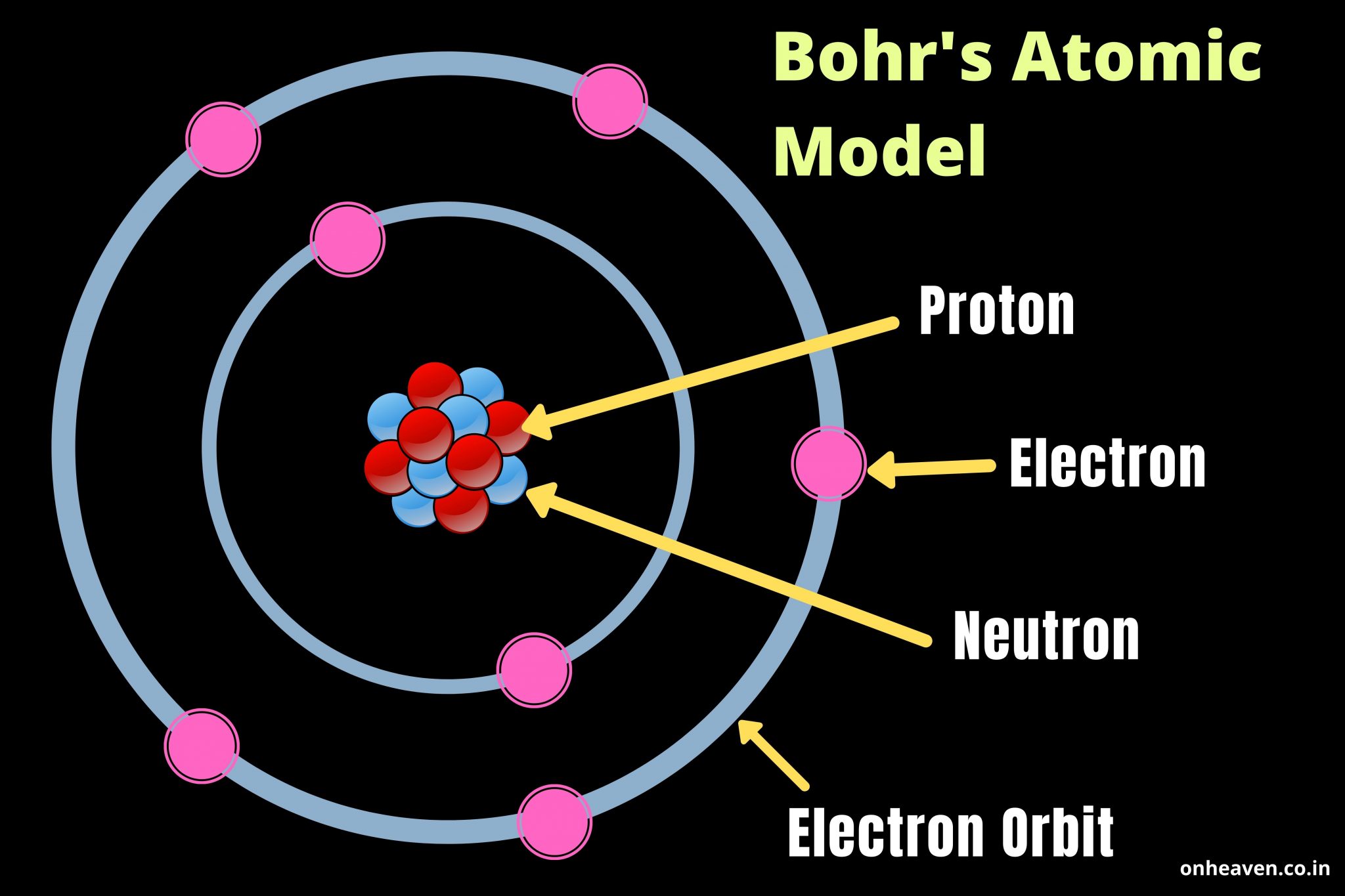

Hydrogen atoms consist of a heavy nucleus with one positively-charged proton around which a single, much smaller and lighter, negatively charged electron orbits. Niels Bohr and quantum theoryīohr was the first physicist to look to the then-emerging quantum theory to try to explain the behavior of the particles inside the simplest of all atoms the atom of hydrogen. For example, how was it possible that the electrons didn't collapse onto the nucleus, since their opposite charge would mean they should be attracted to it? Several physicists tried to answer this question including Rutherford's student Niels Bohr. Some questions, however, remained unanswered. Around this nucleus, the electrons revolved similarly to planets orbiting the sun in our solar system, according to Britannica. According to this model, the atom no longer consisted of just electrons floating in a soup but had a tiny central nucleus, which contained most of the atom's mass. Thomson thought that electrons floated in a positively charged "soup" inside the atomic sphere, according to Khan Academy.ġ4 years later, New Zealand-born Ernest Rutherford, Thomson's former student, challenged this depiction of the atom when he found in experiments that the atom must have a small positively charged nucleus sitting at its center.īased on this finding, Rutherford then developed a new atom model, the Rutherford model. The Bohr model: Journey to find structure of atomsīritish physicist Joseph John Thomson made the first major breakthrough in the understanding of atoms in 1897 when he discovered that atoms contained tiny negatively charged particles that he called electrons.


 0 kommentar(er)
0 kommentar(er)
